
Paraneoplastic retinopathies: an update on pathogenesis, diagnosis and management
Introduction
Paraneoplastic syndromes are rare conditions with a variety of systemic manifestations secondary to an underlying malignancy. Broadly speaking, paraneoplastic syndromes manifest remotely from the site of malignancy and occur secondary to substances produced by cancerous cells—hormones, peptides or cytokines—or immune-mediated molecular mimicry between cancerous antigens and normal tissue (1,2). Paraneoplastic syndromes have been reported in as much as 10% of cancer patients but the prevalence of paraneoplastic syndromes affecting the retina remains unknown (3). The paraneoplastic retinopathies include cancer-associated retinopathy (CAR), melanoma-associated retinopathy (MAR), bilateral diffuse uveal melanocytic proliferation (BDUMP) and paraneoplastic vitelliform maculopathy (PVM). Herein I will review the pathophysiology, clinical findings, multimodal ocular imaging and functional testing, diagnostic testing and treatment paradigm for the various paraneoplastic retinopathies.
CAR
CAR, otherwise known as paraneoplastic autoimmune retinopathy (pAIR) was first described by Sawyer et al. in 1976 in a series of 3 patients with lung cancer suffering from concomitant vision loss and retinal degeneration (4). The retinal degeneration seen in CAR is thought to be secondary to circulating auto-antibodies which cross-react with tumor-specific and retina-specific antigens. This pathophysiology of CAR was proposed by Keltner et al. in 1983 when the group found circulating anti-retinal antibodies in a patient with cervical lymphoma and progressive vision loss (5). The most common primary tumor accounting for the vast majority of cases of CAR is small cell lung cancer, breast cancer or a gynecologic malignancy (6-8). A variety of other solid-tumor associations including non-small cell lung, thyroid, thymus, colon, bladder, pancreatic and hematologic malignancies have also been associated with CAR (6-8). In a series of 209 patients with CAR, Adamus reported an average age of presentation of 65 years with a 2:1 female to male predilection (8). In the same series, the time from cancer diagnosis to onset of CAR varied from weeks to years depending on the type of primary malignancy. That being said, visual symptoms may precede the diagnosis of the underlying malignancy in almost half of patients (6,7).
Vision loss in CAR is typically rapid, painless and may feature light-sensitivity, photopsias, glare, nyctalopia, loss of peripheral and/or central vision, loss of color vision. Visual symptoms in CAR represent widespread dysfunction of both rods and cones though there is a rare variant of CAR which only affects cones (6). Fundus findings in CAR are classically unimpressive relative to the extent of visual symptoms. In more advanced disease there may be retinal vascular attenuation, optic disc pallor and retinal pigment epithelial (RPE) changes. Also variably present are mild vitritis, anterior uveitis, retinal vasculitis and cystoid macular edema (CME) (Figure 1).

Structural and functional multimodal retinal testing can be helpful in establishing the diagnosis of CAR. Optic coherence tomography (OCT) often reveals significant macular outer retinal atrophy with loss of the ellipsoid zone and thinning of the outer nuclear layer (Figure 2) (9). CME and schisis-like changes may also be variably present. Fluorescein angiography (FA) is usually unremarkable except in cases featuring a mild vasculitis in which perivascular leakage may be evident. Fundus autofluorescence (FAF) can be very useful in following the progression of CAR. FAF typically reveals hyperautofluorescence in the region of outer retinal loss essentially from a window defect revealing retinal pigment epithelium (RPE) fluorophores (Figure 1) (10). There may often be a ring of hyperautofluorescence corresponding to the region of outer retinal loss in the macula. With more longstanding diseases, there may also be regions of hypoautofluorescence corresponding to areas of combined outer retinal and RPE death. Visual field abnormalities range from central, cecocentral, paracentral or arcuate defects to ring scotomata or generalized depression (11). Electroretinography (ERG) findings in CAR are representative of global rod and cone dysfunction with attenuated or completely extinguished photopic and scotopic responses (12). ERG may additionally show selective bipolar cell dysfunction in some cases. Despite the array of findings on multimodal retinal diagnostics, there are no specific diagnostic criteria for CAR. The diagnosis thus relies on clinical and ERG findings of retinal degeneration in combination with a diagnosis of a systemic malignancy, circulating anti-retinal antibodies and no alternate etiologies to explain global retinal dysfunction such as a hereditary retinal degeneration.

Laboratory testing in cases of suspected CAR should focus on ruling out masquerades such as syphilis and checking for serum anti-retinal antibodies. The first anti-retinal antibody identified in a patient with small cell lung cancer and CAR was against the 23-kDa antigen, recoverin (13). Recoverin, when present, is now known to be the most sensitive and specific antigen found in CAR. The reported prevalence of anti-recovering antibodies in CAR is 5% (14). Recoverin is a calcium-binding protein found in photoreceptors where it regulates phosphorylation of rhodopsin. Recoverin has been found to be abnormally expressed in tumor cells of patients with CAR. There are numerous other antigens identified in CAR including carbonic anhydrase II (CAII, 30 kDa), transducin- α (40 kDa), α-enolase (46 kDa), arrestin (48 kDa), tubby-like protein 1 (TULP1, 78 kDa) amongst others (14). Adamus and colleagues have proposed that anti-retinal bodies against each of these specific antigens accounts for the phenotypic heterogeneity seen in CAR (8). For example, antibodies against α-enolase target ganglion cells and inner retinal layers and cones while antibodies against transducin- α target rods and ganglion cells which explains the phenotypic variation in an anti- transducin-α retinopathy (loss of night vision, peripheral vision) compared to an anti-α-enolase retinopathy (loss of central and color vision). Both anti-transducin-α retinopathy and anti-α-enolase retinopathy can result in secondary optic nerve atrophy secondary to ganglion cell dysfunction (8).
Presence of anti-retinal antibodies alone is insufficient to diagnose CAR. Anti-retinal antibodies have been detected in normal controls as well as in patients with other ocular diseases (14-16). Additionally, the concordance rate between laboratories checking for anti-retinal antibodies has been shown to be as low as 36% which has been attributed to non-standardized laboratory practices (17,18). There is currently only one Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory in the United States for anti-retinal antibody testing. Recently, Chen and colleagues reported on a series of 14 patients without autoimmune retinopathy whose sera was evaluated for anti-retinal antibodies at this CLIA-certified lab (19). Despite standardized testing protocols, 13/14 patients (93%) tested positive for a median of 5 anti-retinal antibodies. The authors attempted to replicate the results at the Mayo Clinic Neuroimmunology Research Laboratory using similar methodology and found that all 14 patients tested positive for a median of 7 anti-retinal antibodies with a concordance rate of 57%. None of the patients in this study tested positive for recoverin. Given these data, I only test for retinal antibodies in patients with a high suspicion for CAR and other than the presence of antibodies against recoverin, I find positive anti-retinal antibody testing to be of limited clinical utility.
No standardized treatment protocol has been shown to be uniformly effective in cases of CAR. Importantly, treatment of the underlying cancer alone does not prevent visual decline. The basis for the treatment of CAR is long-term immunosuppression with or without adjunctive local steroids (20). In a series of 33 patients with CAR, oral and IV corticosteroids were shown to result in improvement in visual function in 62% of patients (20). In addition to systemic steroids (21), intravenous immunoglobulin (IVIg) (22). and steroid-sparing immunosuppressive agents such as mycophenolate mofetil, azathioprine, cyclosporine, rituximab (anti-CD20 antibody) and alemtuzumab (anti-CD52 antibody) have been reported to be used in cases of CAR with some success (9,23,24).
Ferreyra et al. (15) and Huynh et al. (25) have reported on improvement in visual function in patients with CAR using repeated local corticosteroid injections alone. In both cases systemic immunosuppression was either ineffective or not tolerated. Local steroids thus remain an important adjunct in the management of CAR, especially when CME is present. Despite the use of an aggressive immunosuppressive regimen, vision function in CAR often does not recover and in many cases will continue to decline. Additionally, the impact of a potent immunosuppressive regimen on cancer-related mortality needs to be considered.
MAR
MAR, a subtype of CAR, was first reported in a case report from 1988 wherein a 69-year-old patient developed sudden onset nyctalopia and photopsias 3 years following resection of a cutaneous malignant melanoma (26). Unlike CAR, most cases of MAR have a known history of malignant melanoma and in some cases herald metastatic disease. Most of the initial cases of MAR were found in association with cutaneous melanoma but it has since been described in ciliochoroidal melanoma and mucosal melanoma. In a series of 62 patients with MAR, Keltner et al. found an average latency period of 3.6 years (range, 2 months to 19 years) from the diagnosis of melanoma to onset of MAR (27). The same series found a male preponderance to the development of MAR with the male to female ratio being 4.7:1 despite no such disproportionate predilection of males to cutaneous melanoma.
Typical symptoms of sudden-onset nyctalopia and bilateral photopsias reflect rod dysfunction. Clinical findings and multimodal retinal structural and functional testing are similar to CAR with a few notable exceptions:
- Presenting visual acuity is typically better in MAR than CAR. In the report by Keltner et al., 28/34 patients (82%) had visual acuity of 20/60 or better at presentation (27).
- MAR has historically been thought to be secondary to anti-retinal antibodies affecting bipolar cells. Two independent reports analyzing the sera of patients with MAR identified antibodies against the transient receptor potential cation channel, subfamily M, member 1 [TRPM1, also known as melastatin 1 (MLSN1)] (28,29). TRPM1 is the cation channel responsible light response in retinal ON bipolar cells and is specifically expressed in these bipolar cells (28,29). Mutations in TRPM1 are also responsible for some forms of congenital stationary night-blindness (CSNB) (30), which explains the similarity between features of MAR and CSNB. That being said, several other anti-retinal antibodies have been identified in MAR thus leading to the heterogeneity of the disease as seen in CAR.
- The ERG in MAR is classically electronegative. This features a normal dark-adapted a-wave followed by a markedly depressed b-wave (6). This indicates normal photoreceptor function but disruption of either bipolar cells or transmission between photoreceptors and bipolar cells.
Data on treatment options in MAR are limited to case reports and case series. While there is no consensus treatment regimen for MAR, the initial steps are cytoreduction of metastatic disease with surgery, chemotherapy, and/or radiation followed by IVIg (27). Despite these interventions, the impact on visual outcomes has been underwhelming. In the series from Keltner et al., only 7/62 patients (11%) experienced some visual improvement after some combination of the above therapies. The role of immunosuppression in MAR is unclear but there is naturally a concern that suppression of the tumor surveillance function of the immune system with known metastatic melanoma could worsen survival. Systemic corticosteroids are largely ineffective in MAR though there has been some data to support the use of local steroids especially in cases featuring CME, vitritis or retinal vasculitis. There was recently a case reported by Karatsai et al. in which a patient with MAR was treated with bilateral intravitreal fluocinolone acetonide steroid implants (31). Three years post-implantation, the patient’s vision remained 20/20 in each eye with part resolution of baseline ERG abnormalities.
BDUMP
The term BDUMP was first coined by Barr et al. in 1982 to describe clinical findings in four patients with unusual bilateral proliferation of uveal melanocytes resembling multifocal bilateral uveal melanomas (32). Each case had a concomitant systemic malignancy. The authors noted a case with similar clinical findings reported by Machemer in 1966 (33). BDUMP was further elaborated on by Gass et al. to include vision loss accompanied the following cardinal signs (34):
- Multiple round or oval subtle red patches at the level of the RPE in the posterior fundus (Figure 3).
- Striking pattern of multifocal areas of early hyperfluorescence on FA corresponding to these patches (Figure 3).
- Development of multiple, slightly elevated, pigmented and nonpigmented uveal melanocytic tumors and diffuse thickening of the uveal tract.
- Exudative retinal detachment.
- Rapid progression of cataracts.
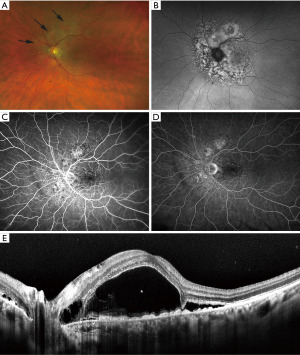
Gass et al. noted that the first two cardinal signs often antedate the following three. Since then, dozens of cases of BDUMP have been reported with most patients being diagnosed in the sixth and seventh decade (34). The majority of associated malignancies in women are urogenital cancers, while in men, lung cancers are most common. The diagnosis of BDUMP often antedates the diagnosis of a systemic malignancy in about half of affected patients with the average survival after the diagnosis of BDUMP being 15 months (35-37).
In addition to the cardinal features described above, eyes with BDUMP have been noted to have a “giraffe pattern” to their fundus reflective of circular or polygonal patches of RPE atrophy with surrounding orange zones of RPE hypertrophy. The patches of RPE atrophy are responsible for the characteristic window defects seen on FA. OCT similarly shows areas of RPE hypertrophy and RPE loss. FAF will typically show hypoautofluorescence in region of RPE atrophy with intervening hyperautofluorescence in region of RPE hypertrophy (Figure 3).
While the mechanism is unclear, the primary tumor in BDUMP stimulates proliferation of uveal melanocytes. One hypothesis is that the primary tumor secretes melanocytic growth factors. This is supported by the observation that about 25% of patients with BDUMP develop skin or mucous membrane hyperpigmentation (38). Additionally, one study found that serum and plasma from some patients with BDUMP caused proliferation of cultured human melanocytes (39).
A review of 68 cases of BDUMP from the literature published in 2016 showed modest visual improvement with various therapeutics (40). In 9/68 patients, treatment was directed only at the primary malignancy which resulted in improved visual function in 5/9 patients. Most patients in the series received local or systemic corticosteroids either alone or in combination with other modalities such as anti-vascular endothelial growth factor (anti-VEGF) therapy or radiation. These therapeutic options did not appear to show any benefit. As of 2020 there have been approximately 20 published cases of BDUMP treated with plasmapheresis of which 13 were reported to have some improvement in visual function (35). Given the high rate of mortality in BDUMP, long-term data on the efficacy of any specific treatment modality is lacking.
PVM
PVM has been described under multiple names including paraneoplastic vitelliform retinopathy and acute exudative paraneoplastic polymorphous vitelliform maculopathy (AEPPVM). PVM is a form of vitelliform disease characterized by acute, often bilateral vision loss with associated multifocal vitelliform retinal lesions occurring in the setting of a known malignancy (Figure 4). It was initially thought to occur most often in patients with a melanoma, but has also been observed in patients with other malignancies as well (41,42). The diagnosis of PVM may follow the diagnosis of the primary tumor by years. That being said, PVM not only appears to correlate with the presence of metastatic disease but the severity of PVM may correlate with the metastatic disease burden (41-43).

Patients may complain of vision loss, photopsias, metamorphopsia, glare, halos and/or nyctalopia. Funduscopy typically reveals bilateral multifocal yellow-orange vitelliform lesions with associated low serous retinal detachments. OCT reveals an accumulation of hyperreflective material in the subretinal space as well as hyporeflective serous fluid (Figure 4). With time, the hyperreflective material pools inferiorly within a pocket of serous retinal detachment. FA may reveal early blockage and late staining but, in many cases, FA is unremarkable (41-43). Indocyanine green angiography (ICGA) may reveal hyperfluorescent spots corresponding to the vitelliform lesions (44). FAF reveals areas of hyperautofluorescence corresponding to the vitelliform material clinically and on OCT (Figure 4). The clinical features may closely resemble a bestrophinopathy and it should be noted that an idiopathic acute exudative polymorphous vitelliform maculopathy may be indistinguishable on funduscopy from the paraneoplastic variant (45).
The pathophysiology of PVM is unclear but mechanistically it would seem that there is a soluble factor produced either by the primary tumor or by the immune system in response to the tumor which impacts RPE cell function (6). Several different circulating anti-retinal and anti-RPE antibodies have been described in patients with PVM but none appear to be uniformly present in all such patients (6). Treatment involves management of the underlying malignancy. Mueller et al. reported on a case of a patient who presented with PVM 1 month following the diagnosis of metastatic melanoma (43). The metastatic disease was treated with immunotherapy, radiosurgery and radiation over a 48-month period and interestingly the maculopathy was noted to improve or worsen in parallel with the metastatic disease burden.
Conclusions
The paraneoplastic retinopathies are a heterogeneous group of conditions with overlapping clinical features and pathophysiology. Establishing a diagnosis can be very challenging and is reliant on multimodal retinal structural and functional testing and clinical suspicion. Early diagnosis may however lead to improved chances of survival in cases where the diagnosis of an underlying malignancy has not yet been discovered. Novel insights into disease pathophysiology may allow for more targeted therapeutics and additional research on this topic is warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kareem Moussa) for the series “The Retina and Systemic Disease” published in Annals of Eye Science. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-23-14/coif). The series “The Retina and Systemic Disease” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543-54. [Crossref] [PubMed]
- Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 2010;85:838-54. [Crossref] [PubMed]
- Kumar VA, Abbas A, Fausto N. Neoplasia. In: Robbins and Cotran Pathologic Basis of Disease. 7th Edition. Philadelphia, PA: Elsevier Saunders; 2005:333-5.
- Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81:606-13. [Crossref] [PubMed]
- Keltner JL, Roth AM, Chang RS. Photoreceptor degeneration - A possible autoimmune disease. Arch Ophthalmol 1983;101:564-9. [Crossref] [PubMed]
- Rahimy E, Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Surv Ophthalmol 2013;58:430-58. [Crossref] [PubMed]
- Singh D, Tripathy K. Cancer associated retinopathy. Treasure Island (FL): StatPearls Publishing; 2024.
- Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 2009;8:410-4. [Crossref] [PubMed]
- Mahdi N, Faia LJ, Goodwin J, et al. A case of autoimmune retinopathy associated with thyroid carcinoma. Ocul Immunol Inflamm 2010;18:322-3. [Crossref] [PubMed]
- Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina 2012;32:1385-94. [Crossref] [PubMed]
- Ohguro H, Yokoi Y, Ohguro I, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol 2004;137:1117-9. [Crossref] [PubMed]
- Matsui Y, Mehta MC, Katsumi O, et al. Electrophysiological findings in paraneoplastic retinopathy. Graefes Arch Clin Exp Ophthalmol 1992;230:324-8. [Crossref] [PubMed]
- Thirkill CE, FitzGerald P, Sergott RC, et al. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med 1989;321:1589-94. [Crossref] [PubMed]
- Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina 2014;34:827-45. [Crossref] [PubMed]
- Ferreyra HA, Jayasundera T, Khan NW, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol 2009;127:390-7. [Crossref] [PubMed]
- Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol 2011;129:415-20. [Crossref] [PubMed]
- Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol 2013;131:113-5. [Crossref] [PubMed]
- Adamus G, Wilson DJ. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol 2009;147:557-author reply 557-8. [Crossref] [PubMed]
- Chen JJ, McKeon A, Greenwood TM, et al. Clinical Utility of Antiretinal Antibody Testing. JAMA Ophthalmol 2021;139:658-62. [Crossref] [PubMed]
- Dutta Majumder P, Marchese A, Pichi F, et al. An update on autoimmune retinopathy. Indian J Ophthalmol 2020;68:1829-37. [Crossref] [PubMed]
- Jacobzone C, Cochard-Marianowski C, Kupfer I, et al. Corticosteroid treatment for melanoma-associated retinopathy: effect on visual acuity and electrophysiologic findings. Arch Dermatol 2004;140:1258-61. [Crossref] [PubMed]
- Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol 1999;117:471-7. [Crossref] [PubMed]
- Espandar L, O'Brien S, Thirkill C, et al. Successful treatment of cancer-associated retinopathy with alemtuzumab. J Neurooncol 2007;83:295-302. [Crossref] [PubMed]
- Davoudi S, Ebrahimiadib N, Yasa C, et al. Outcomes in Autoimmune Retinopathy Patients Treated With Rituximab. Am J Ophthalmol 2017;180:124-32. [Crossref] [PubMed]
- Huynh N, Shildkrot Y, Lobo AM, et al. Intravitreal triamcinolone for cancer-associated retinopathy refractory to systemic therapy. J Ophthalmic Inflamm Infect 2012;2:169-71. [Crossref] [PubMed]
- Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol 1988;106:307-11. [Crossref] [PubMed]
- Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol 2001;21:173-87. [Crossref] [PubMed]
- Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One 2011;6:e19911. [Crossref] [PubMed]
- Dhingra A, Fina ME, Neinstein A, et al. Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J Neurosci 2011;31:3962-7. [Crossref] [PubMed]
- Nakamura M, Sanuki R, Yasuma TR, et al. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis 2010;16:425-37. [PubMed]
- Karatsai E, Robson AG, Taylor SRJ. Outcomes Associated With Sustained-Release Intraocular Fluocinolone Implants in a Case of Melanoma-Associated Retinopathy Treated Without Systemic Immunosuppression. JAMA Ophthalmol 2019;137:564-7. [Crossref] [PubMed]
- Barr CC, Zimmerman LE, Curtin VT, et al. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms. A recently recognized syndrome. Arch Ophthalmol 1982;100:249-55. [Crossref] [PubMed]
- Machemer R. Zur pathogenese des flachenhaften malignen melanoms. Klin Monatsbl Augenheilkd 1966;149:641-52. [PubMed]
- Gass JD, Gieser RG, Wilkinson CP, et al. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol 1990;108:527-33. [Crossref] [PubMed]
- Weppelmann TA, Khalil S, Zafrullah N, et al. Ocular Paraneoplastic Syndromes: A Critical Review of Diffuse Uveal Melanocytic Proliferation and Autoimmune Retinopathy. Cancer Control 2022;29:10732748221144458. [Crossref] [PubMed]
- Parakh S, Maheshwari S, Das S, et al. Presumed bilateral diffuse uveal melanocytic proliferation - A case report and review of literature. Am J Ophthalmol Case Rep 2022;27:101582. [Crossref] [PubMed]
- Rahimy E, Coffee RE, McCannel TA. Bilateral diffuse uveal melanocytic proliferation as a precursor to multiple systemic malignancies. Semin Ophthalmol 2015;30:206-9. [Crossref] [PubMed]
- O'Neal KD, Butnor KJ, Perkinson KR, et al. Bilateral diffuse uveal melanocytic proliferation associated with pancreatic carcinoma: a case report and literature review of this paraneoplastic syndrome. Surv Ophthalmol 2003;48:613-25. [Crossref] [PubMed]
- Miles SL, Niles RM, Pittock S, et al. A factor found in the IgG fraction of serum of patients with paraneoplastic bilateral diffuse uveal melanocytic proliferation causes proliferation of cultured human melanocytes. Retina 2012;32:1959-66. [Crossref] [PubMed]
- Moreno TA, Patel SN. Comprehensive Review of Treatments for Bilateral Diffuse Uveal Melanocytic Proliferation: A Focus on Plasmaphereis. Int Ophthalmol Clin 2017;57:177-94. [Crossref] [PubMed]
- Aronow ME, Adamus G, Abu-Asab M, et al. Paraneoplastic vitelliform retinopathy: clinicopathologic correlation and review of the literature. Surv Ophthalmol 2012;57:558-64. [Crossref] [PubMed]
- Al-Dahmash SA, Shields CL, Bianciotto CG, et al. Acute exudative paraneoplastic polymorphous vitelliform maculopathy in five cases. Ophthalmic Surg Lasers Imaging 2012;43:366-73. [Crossref] [PubMed]
- Mueller CM, Hojjatie SL, Lawson DH, et al. Clinical Correlation between Acute Exudative Polymorphous Paraneoplastic Vitelliform Maculopathy and Metastatic Melanoma Disease Activity: A 48-month Longitudinal Case Report. Ocul Immunol Inflamm 2022;30:330-7. [Crossref] [PubMed]
- Torres-Costa S, Penas S, Carneiro Â, et al. Idiopathic Acute Exudative Polymorphous Vitelliform Maculopathy: Insight into Imaging Features and Outcomes. Case Rep Ophthalmol Med 2020;2020:7254038. [Crossref] [PubMed]
- Barbazetto I, Dansingani KK, Dolz-Marco R, et al. Idiopathic Acute Exudative Polymorphous Vitelliform Maculopathy: Clinical Spectrum and Multimodal Imaging Characteristics. Ophthalmology 2018;125:75-88. [Crossref] [PubMed]
Cite this article as: Thomas AS. Paraneoplastic retinopathies: an update on pathogenesis, diagnosis and management. Ann Eye Sci 2024;9:2.


